News
Meet an Alien: Xenobiology is coming of age!
Xenobiology has now matured intellectually and experimentally as a scientific discipline. It is expected to provide us with a good starting point and platform for current and future attempts to fundamentally remake life or even create life forms from scratch!
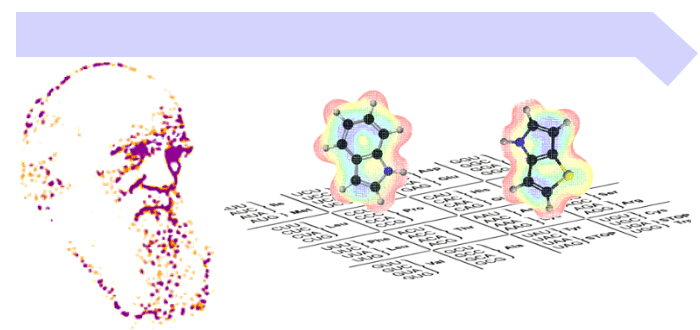
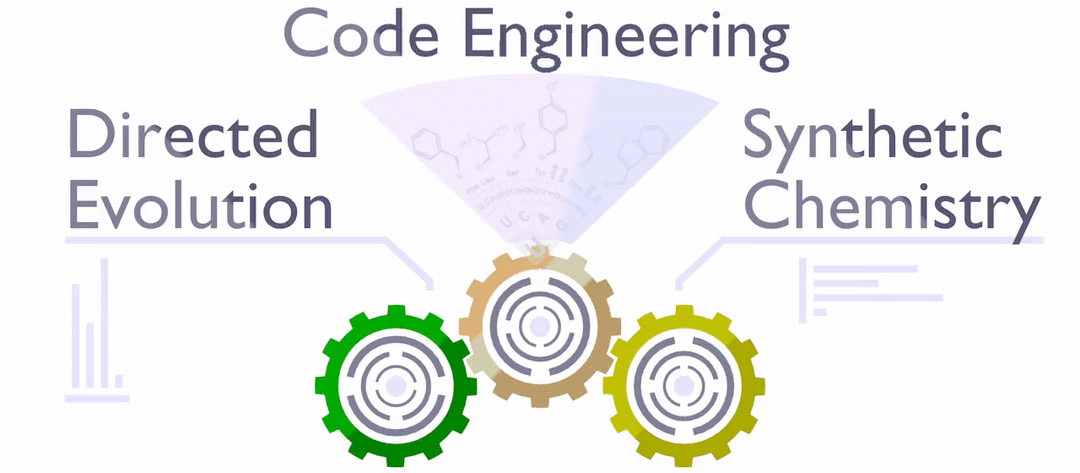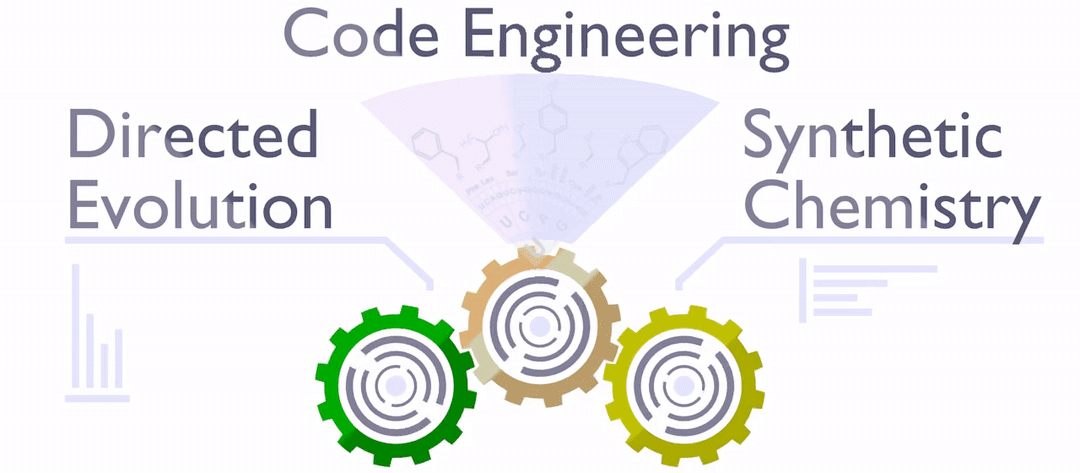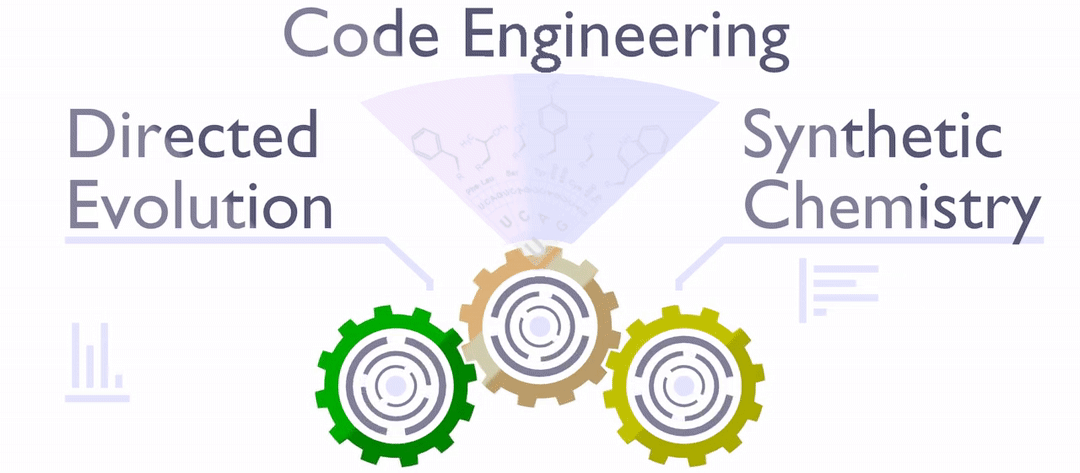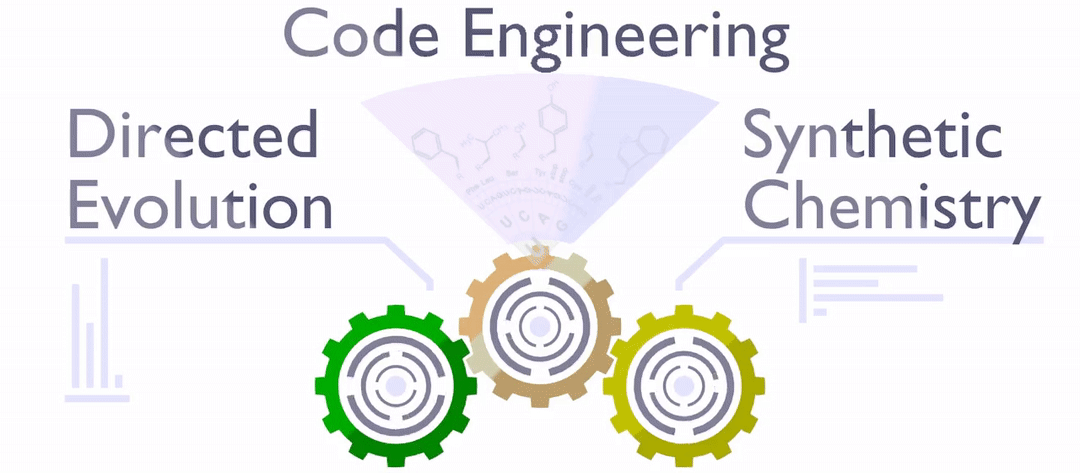
With the publicly available Special Collection Xenobiology 2020, we will get much clearer ideas and inspiration on how to completely change the chemical implementation of life (both top-down and bottom-up), which means to synthetically (re-)design every single element of life (see figure below).
The diversity in nature usually leads to the creation of species that are "reproductively isolated" (classic definition of species). In Xenobiology however, engineered diversification (top-down) and de novo (from scratch) synthesis (bottom-up) leads to the creation of contained parallel biological worlds in genetic isolation.
Since coding is an essential feature of life, we expect that real artificial, synthetic (alien) life will be equipped with novel genetic codes! You can read more about this in our editorial for Special Collection of ChemBioChem Xenobiology 2020.
However, it could be misleading when talking about “creating aliens”. Extraterrestrials might already exist somewhere in space (or maybe on Earth, hidden from us). When we talk about aliens in everyday life, we always have extraterrestrials in mind. However, the ‘aliens’ of Xenobiology are no extraterrestrials. They are our creations!
Our recent proposal of the “Alanine World” might be helpful (to some extent) in this regard. When we talk about our world as the Alanine-World, it helps us to clearly demarcate it from ‘XB aliens’ as well as possible aliens of Extraterrestrial Biology and Astrobiology. But who guarantees that extraterrestrial or ‘hidden’ aliens are not based on the Alanine World as well? Maybe we are living in the best of all possible worlds. Who knows?
This is a good point to stop and go back to the boring mainstream science and do something in the laboratory…
Genetic code engineering meets nanotechnology
Selective targeting of cellular receptors is the main strategy to block the influenza virus, which is adapted by viruses and other parasites to infect the host cell. This event could be blocked or significantly disrupted by the development of specific binders that act on either cellular receptors or by blocking pathogen recognition proteins. This is exactly what we have been doing in cooperation with various Berlin groups over the past six years.
For further information, take a look at Science Daily and University of Manitoba press release.
Original paper: Nature Nanotechnology, 2020; DOI: 10.1038/s41565-020-0660-2
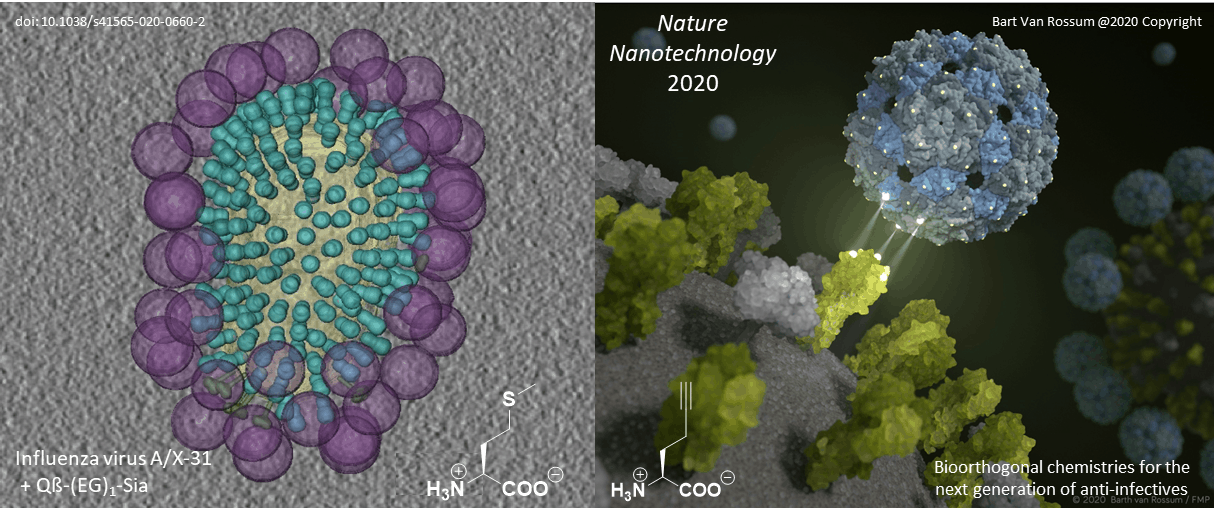
Proteins with well-characterized three-dimensional structures are ideal frameworks for nanotechnology. Their defined and rigid three-dimensional structure enables the positioning of ligands with an almost perfectly defined geometry. Most importantly, our genetic code engineering enables us to endow target protein scaffolds and their assemblies (such as viral capsids) with bioorthogonal chemical functionalities provided by non-canonical amino acids (ncAAs).
...or why did nature choose the genetic code with "only 20” canonical amino acids for ribosomal protein synthesis?
There seem to be several explanations for why nature chose 20 amino acids (as basic building blocks - monomers - for proteins) within the (almost) universal genetic code. Our contribution is a model of the "Alanine World", which is proposed from the viewpoint of peptide and protein chemistry. The core of our considerations is the choice of secondary structural elements for building up the protein scaffolds. This also determines the choice of amino acid monomers. Dominant secondary structures in life as we know it are α-helices and β-sheets, which are mainly made up of alanine derivatives (from a chemical point of view). That is why Vladimir coined the term "Alanine World". In this model, the selection of monomers (amino acids) for such secondary structural elements is rather limited. For example, chemical propensities for α-helix or β-sheet determine exactly which side chains of alanine derivatives are suitable and which are not.
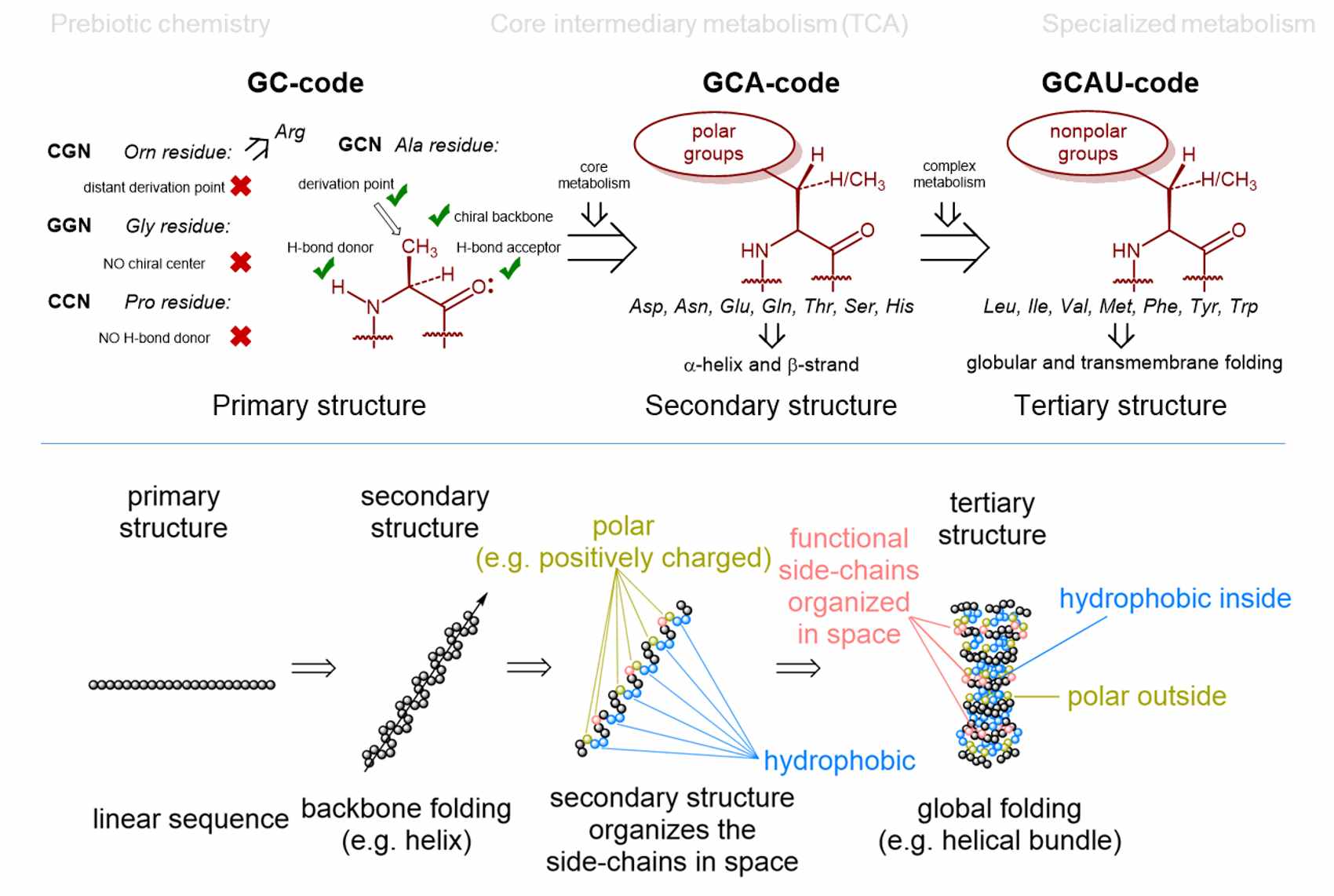
See our current papers for further details and comprehensive explanations as well as possible biotechnological relevance and impact.
Curiously, the numbers 3, 4, 64 and 20 in the flow of genetic information also seem to be magically attractive to quantum physicists. These numbers are obtained using strict quantum mechanical algorithms for the flow of genetic information (DNA -> RNA -> protein). The result is 4 letters (AUGC), 64 triplets and 20 monomers (amino acids) as optimal numbers.
That said, different scientific approaches allow the conclusion that the structure of the genetic code is deterministic. Does this mean that the existing living world is "the best of all possible" worlds? If so, the question arises whether it is worth at all to create an alternative to extant life?
Computational design takes over!
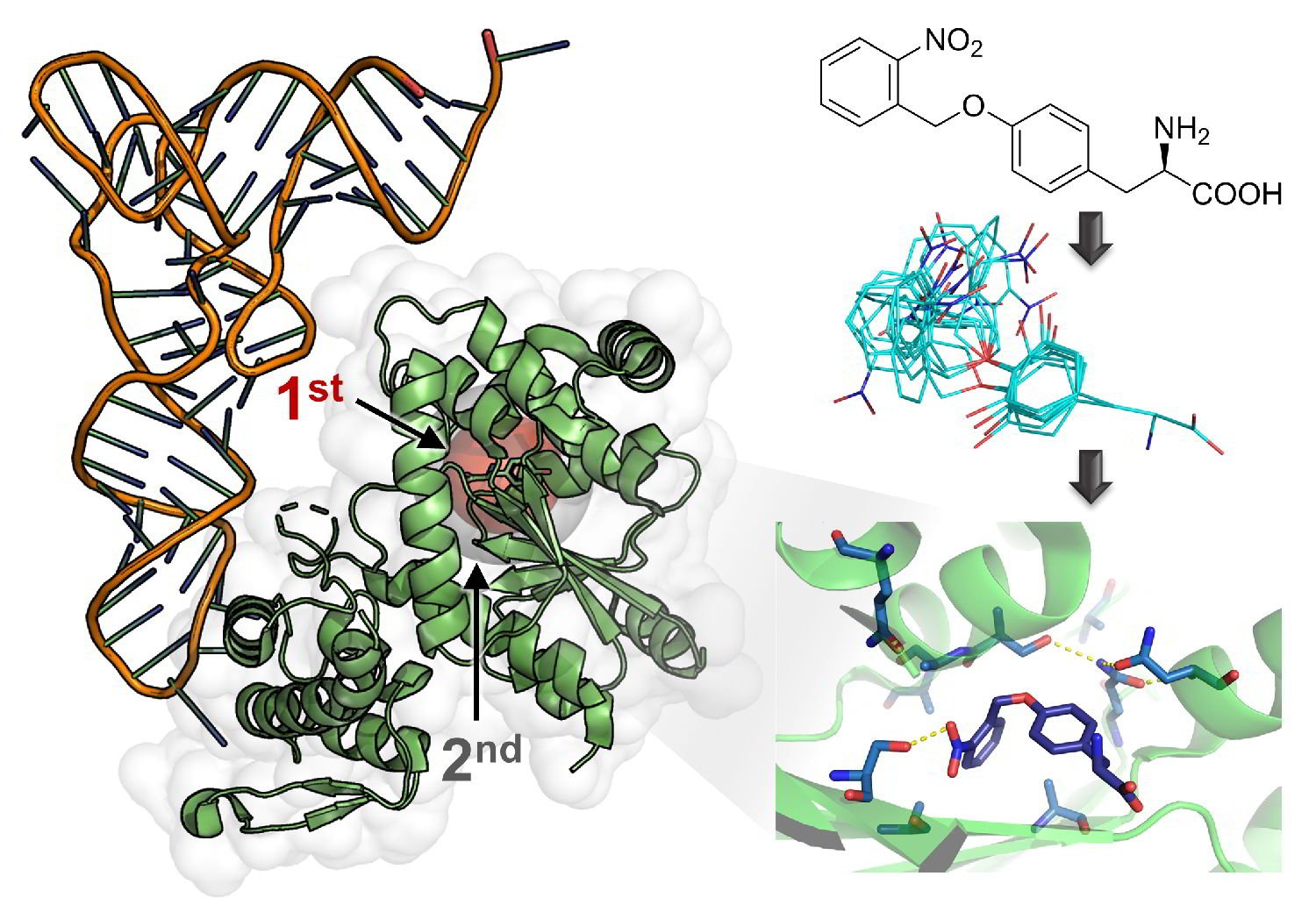
Computer-aided design provided an enzyme that surpassed all related enzymes generated by classical engineering methods.
Computational design and machine learning in the process of evolving highly active artificial enzymes is becoming an indispensable tool and is likely to completely replace classical approaches in the near future.
Here is our contribution:
In vivo Azulenyl-alanine incorporation into proteins by directed evolution of orthogonal enzymes
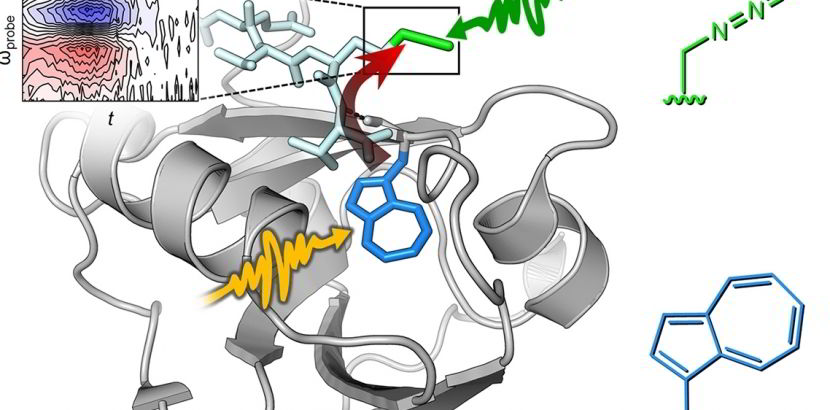 For more Information, see:
For more Information, see:
Excellent location in the land of ideas


Our project Xenoglue has recieved a award for its unique apporach and great potential. The price was awarded from the initiative "Deutschland-Land der Ideen" (Germany-land of ideas).
We are moving to Canada
The head of the group, Nediljko Budisa, has accepted a position at the University of Manitoba, located in Winnipeg, province Manitoba, Canada. We will officially move there starting from the 01.10.2018.


Prize for Xenoglue Team!

The technology of Xenoglue, which originates from our group, won the Science4Life start-up competition. Two represantatives of the Xenoglue-team, Christian Schipp and Tobias Schneider, took the award on the 13.03.2018 and the price money of 1000€. Before that they took part in a two day workshop about startup management. Xenoglue strives to develop a bio degradable glue, which is based on the natural example of the mussel and is intended for usage in wound healing. Scientist have studied the subject of the mussels adhesion mechanism for decades. (See news from 01.08.2017)
BIOMOD 2017: Gold winning MultiBrane team back from San Francisco!

The team MultiBrane of the tu project "iGEM Synthetic Biology" 2017 belongs to the big winners of this year's BIOMOD competition. The research objective was to create a multifunctional membrane for waste water treatment with a particular focus on microplastics & micropollutant removal. The results were very compelling and achieved high visibility in San Francisco, where the BIOMOD jamboree took place at UCSF University. Especially in three categories, MultiBranes convinced the international judges and received a gold medal for their project work:
> 3rd place in the best project website and
> 3rd place in the best video category
We would therefore like to express our sincere thanks to Saba Nojoumi and Franz-Josef Schmitt for their dedicated mentoring and support respectively. We also thank Hannah Aring and Nikolaj Koch for their work within the scope of tu projects. With this in mind, we congratulate again and wish all the best to all team members and look forward to the next exciting BIOMOD and iGEM projects in the future.
The project results can be found on the team Website.
Towards biological underwater superglue!

Regenerative medicine urgently needs biocompatible adhesives suitable for therapeutic use in the treatment of small bone fractures. Such an adhesive should allow rapid bonding of the bone fragments without the need for the attachment of plates and screws. The use of biological glue from marine mussels (so-called mussel-adhesive proteins, MAPs) - as potential environmentally-friendly bio-inspired adhesives - could be a solution to this problem. In nature, mussel secrets adhesive proteins and efficiently adheres to stone and other inorganic surfaces and even to man-made products such as metal and plastic (e.g. Teflon) materials. The secret of these bio-glues is the presence of catechol groups in the side chain of the non-proteinogenic amino acid L-dopa (L-3,4-dihydroxyphenylalanine) which is produced post-translationally by tyrosine hydroxylation and is capable of surface adhesion.
However, isolation of such bio-glues from natural sources is expensive, inefficient, and it is not possible to produce large amounts of a homogeneous product by imitating post-translational modification machineries. On the other hand, current synthetic chemical or biotechnological synthesis pathways are not efficient. For these reasons, we have endeavored to solve this problem by applying an orthogonal amino acid translation to develop a new concept to produce DOPA-rich underwater adhesive proteins from marine mussels by genetic code expansion.
By using computational design and genetic selection methods, a novel Methanocaldococcus jannaschii tyrosyl-tRNA synthetase-based enzyme was engineered. It activates the photocaged DOPA derivative ortho-nitrobenzyl-Dopa (ONB-DOPA) for incorporation into proteins in response to amber stop codons through orthogonal translation. In this way, mussel proteins are equipped with ONB-DOPA at multiple sites, which introduces spatiotemporal control over their adhesive properties. Exposure to UV light triggers cleavage of the ONB photocage, liberating the adhesive catechol side chain of DOPA. This strategy provides new ways for producing DOPA-based wet adhesives for application in industry and biomedicine with the potential to revolutionize bone surgery and wound healing.
Public resonance:Synthetic alienation of microbes - "thawing" of the "frozen" code
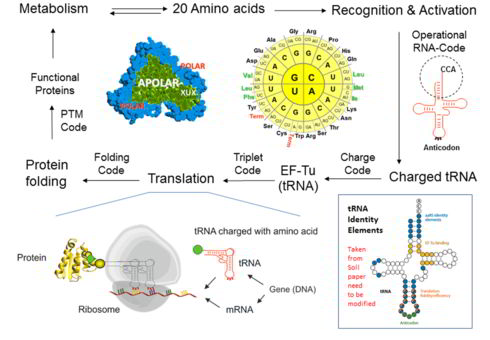
It is assumed that the genetic code experienced a transition from an ambiguous to a well-defined ("frozen") code, as we know it. During this evolution, the protein translation, which first operated with few amino acids and produced the statistical proteins, developed into a highly accurate machine capable of producing very specific proteins, based on the 20 canonical amino acids. Since the genetic code operates well in many ways (e.g. error minimization, protein folding, and metabolic integration), the fundamental question is, what are the barriers to attempts to change it experimentally? How can they be overcome? We have tried to find an answer and written an essay that is now published in the Biotechnology Journal (link) with a model that has allowed us to provide "entry points" for noncanonical amino acids in the ribosome protein synthesis cycle as shown above.